Sterile processing for optimum safety in the clinic AEMP
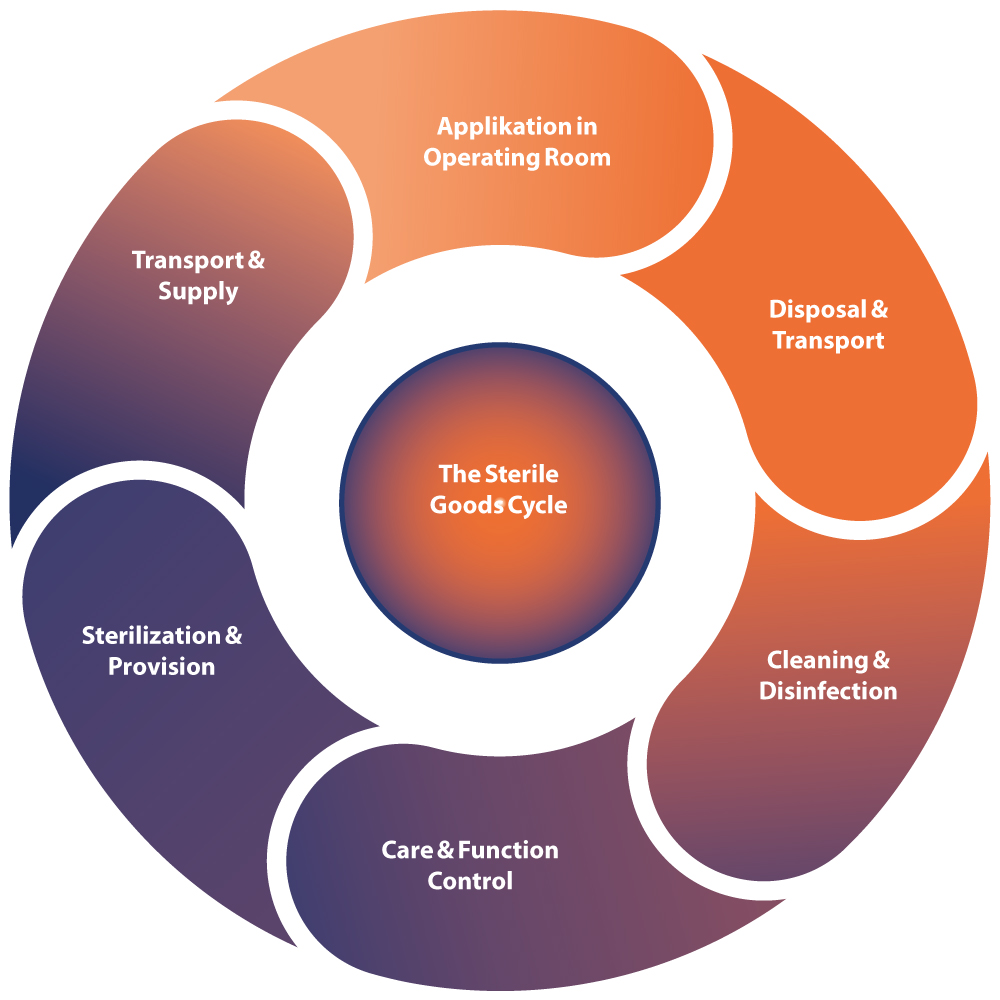
Improving the Sterile Goods Processing Process in Hospitals
Enhancing process security and reducing undesired costs through a comprehensive examination of the sterile goods processing process in hospitals. Cleaning, disinfection, and sterilization are crucial steps to prevent the transmission of germs or pathogens and ensure the safety of medical instruments.
The Medical Devices Implementation Act (MPDG) and the Medical Devices Operator Ordinance (MPBetreibV) legally stipulate that only hygienically flawless and professionally processed medical instruments may be used on patients.
One of the main causes of germ and pathogen transmission often lies in the sterile goods processing process.
In Germany, approximately 30,000 to 35,000 nosocomial infections with MultiResistant Enterobacteria (MRE) occur annually. 1,500 cases, which equates to 0.3% of all nosocomial infections in Germany, are caused by multi-resistant pathogens that are resistant to almost all classes of antibiotics.
According to the Robert Koch Institute (RKI), there are a total of approximately 400,000 to 600,000 nosocomial infections per year in Germany, causing approximately 10,000 to 20,000 deaths. In Europe, approximately 91,000 deaths are attributed to nosocomial infections annually.
The causes of nosocomial infections and MRE can include the sterile goods processing process, where the quality of the instruments plays a significant role in transmission.
- Tensile cracks and wear
- Corrosion, chroming, and surface deposits
- Functional testing in the Department of Medical Device Processing (AEMP) and in the Operating Room (OR)
- Steam quality of steam sterilizers
- Cleaning performance of cleaning and disinfection devices (RDG) / container washing machines (CWA)
- Quality of process water
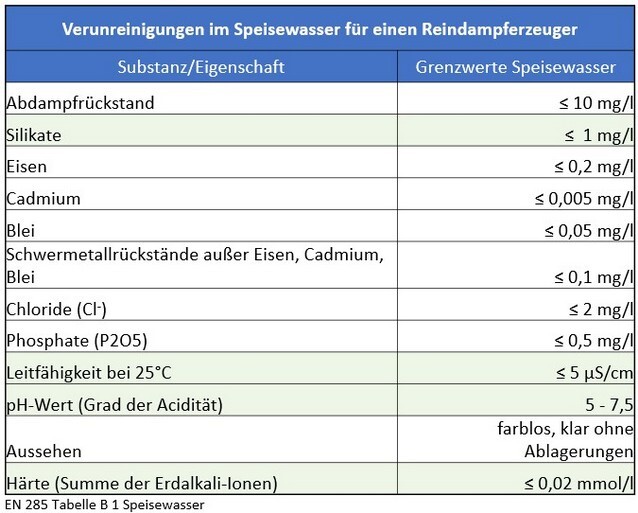
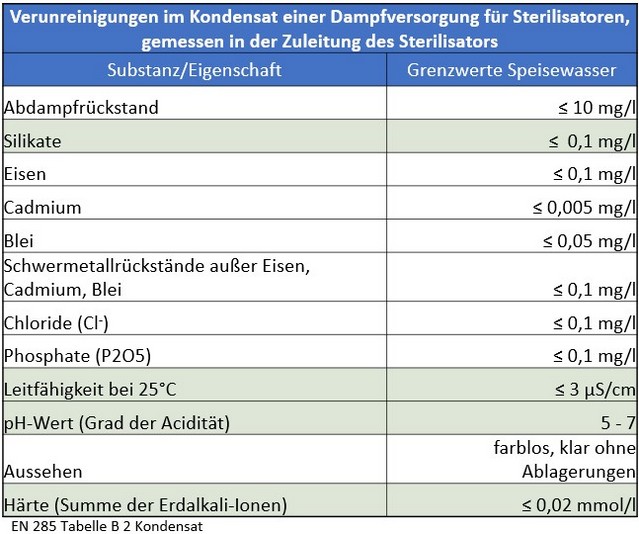
However, long-standing practical experience shows that the threshold values defined in the current standard for feed water and condensate still lead to premature wear of systems, sterile goods, and instruments. They do not meet the hygienic requirements for an uncontaminated, sterile medical product and do not adequately protect either patients or investments.
Water treatment is therefore a crucial part of the instrument cycle. Not all water is the same, and neither is all steam.
The sterilization process and all associated aspects of media generation are fundamental to the overall process. The generated water is not only used for producing pure steam but also for rinsing and cleaning processes.
The interaction of various technical components poses a challenge and requires precise coordination and consideration of the entire process, including provision, use, and reprocessing of the instrumentarium.
Insufficient knowledge about the overall process, the condition of the entire system including the piping network, equipment technology, and treatment capacities lead to problems and thus damages to the treated goods. This poses a risk to patient safety.
Steam sterilization is an extremely sensitive and demanding part of medical device processing. Process water and steam in the sterilizers (autoclaves) have direct effects on the instruments and represent a latent high hygienic and costly process risk, which indeed leads to significant risks to patient safety.
In daily practice, it is evident that the threshold values defined in the current DIN EN 285 for feed water and condensate in this process prematurely lead to quality deficiencies in equipment technology, components, and the overall system of sterile goods processing.
There is currently no legally binding standard for the planning of water treatment for the sterilization of instruments. The planning and design are carried out in accordance with or based on the application of DIN EN 285.
In addition to the negative impacts on patient safety, contaminated or defective medical devices also incur high costs for new acquisitions and/or repairs.
For hospitals with at least 4 surgical disciplines and various bed capacities, the table below illustrates possible acquisition costs for surgical instruments as well as average repair costs of the instrumentation:
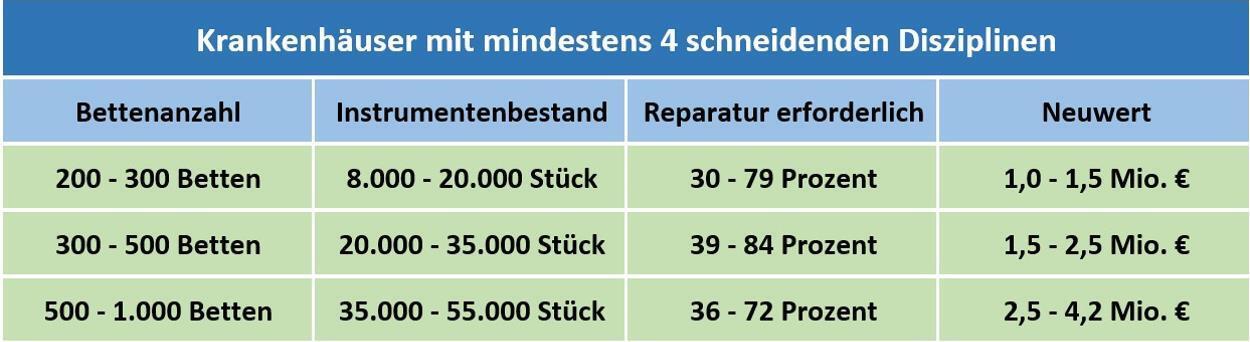
Overview of Instrument Needs and Cost Commitment
Basis: Approximately 200 inventory analyses in the years 2002 – 2021

- Are the costs for repairs and replacement services of medical devices still in an appropriate proportion within the clinic?
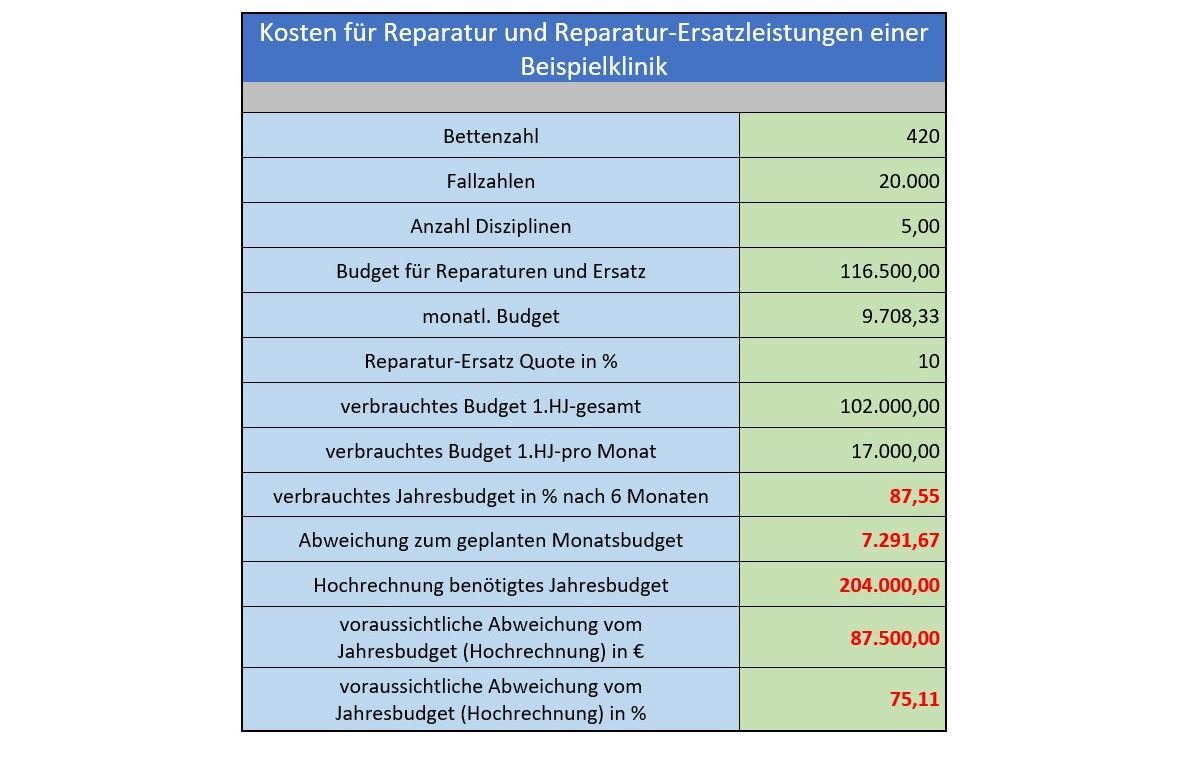
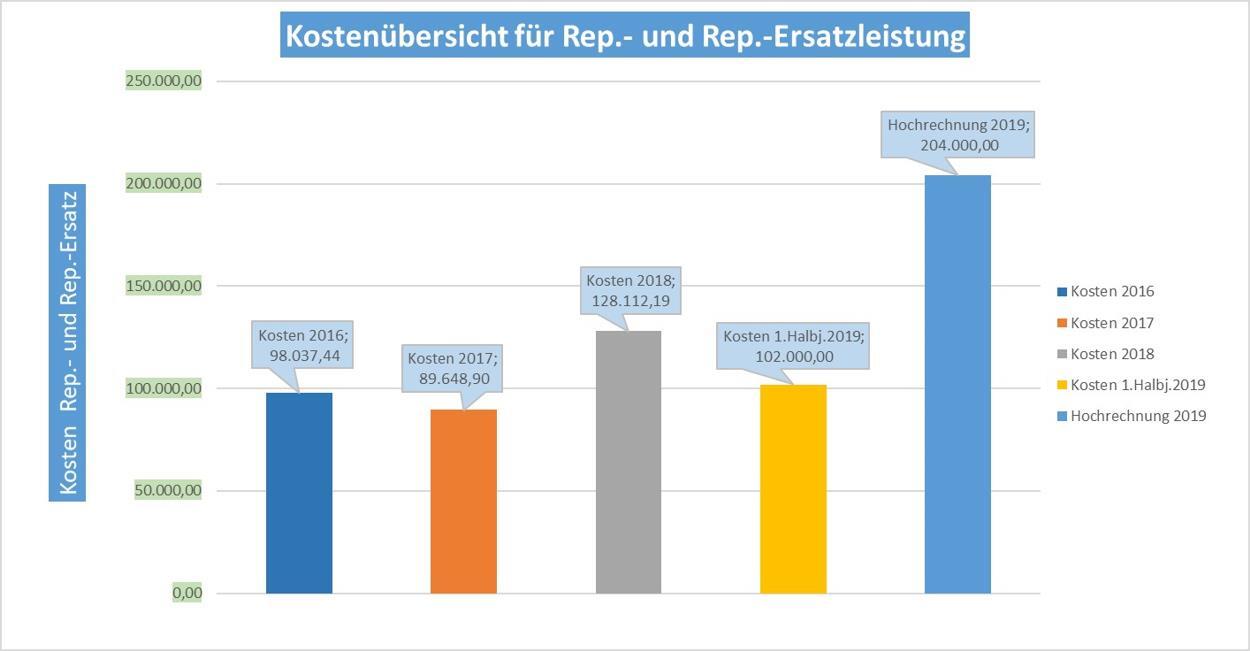
- The allocated budget is nearly depleted after 6 months.
- A significant exceeding of the annual budget is anticipated.
An investigation must be conducted to determine the factors contributing to this situation.
A significant deviation from the budgeted costs indicates issues with handling the instrumentation, processing, and associated media and their generation. The above results highlight an urgent need for action.
Damage to the instruments shortens their lifespan from approximately 15 years to less than 8 years with poor media quality. Poor media quality is directly related to the operating costs of the instruments.
The entire hospital’s sterile goods processing process should be thoroughly assessed and analyzed immediately. This can help avoid a potential total loss of existing instrumentation and equipment technology, thereby not substantially compromising patient safety.
Through the example described above, it becomes evident that continuous monitoring of the sterile goods processing process is essential to prevent damage to instruments and equipment technology. This ensures patient safety and avoids unforeseen costs.
- Who monitors the sterile goods processing process and detects process errors early?
- Who assesses whether the process is still in an optimal condition?
Responsibilities and Processes in a Medical Device Reprocessing Unit (MDRU)
The previous considerations regarding the costs of repairs and replacement services for instrumentation have already underscored the high importance of media quality. Ensuring this quality and all associated processes falls under the responsibility of the following designated departments:
- Management and staff of the MDRU (formerly known as CSSD)
- Hygiene officers and hygiene specialists
- Equipment and building technicians
- Operating rooms and functional areas
- Logistics management
as well as
- Chemical suppliers, instrument manufacturers, equipment technology manufacturers, and water treatment specialists.
The management plays a crucial role in this regard. Their responsibility lies in the successful economic management of the healthcare facility while adhering to quality criteria to ensure instrument and patient safety. Given the specific personal liability risk as operators of healthcare facilities according to the Medical Devices Implementation Act (MPDG), the CEO and all the aforementioned groups are responsible for ensuring the proper reprocessing of medical devices.
Negative impacts due to improperly reprocessed instruments, such as delays or cancellations of surgeries, which have negative economic implications, are to be avoided.
The numerous stakeholders highlight the complex interplay between water treatment specialists, equipment technology suppliers, end-users, and other involved parties within the sterile goods processing process. The complex responsibilities and existing interfaces require interdisciplinary collaboration among the involved groups.
Objectives / Process Requirements
Ensuring the necessary media safety in process water treatment and thus optimally minimizing the process risk for the reprocessing of medical devices, as well as reducing risks to patient safety to a minimum, represent the primary goal. The design of the process also takes into account the cost-effectiveness of sterile goods processing by identifying savings potentials considering individual obstacles and structural barriers.
The focus is on avoiding and preventing costs for the refurbishment or acquisition of equipment and facility technology, as well as the renewal of entire supply systems.
How should the sterile goods processing process be assessed to achieve the stated objectives?
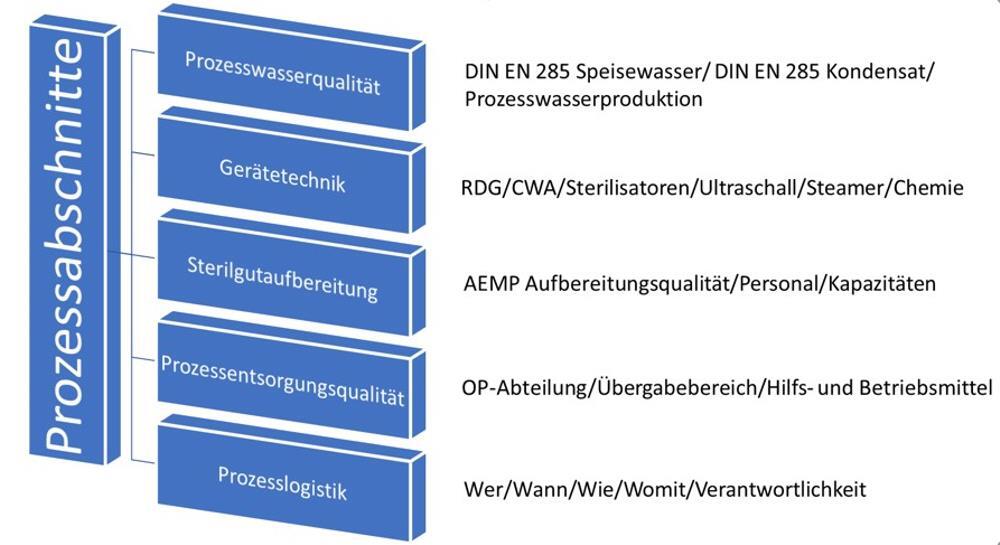
Starting with the process water, the following points are crucial to consider in the overall assessment for planning a new facility or evaluating an existing water treatment system:
- What are the intended uses of the treated water?
- Are there standards or guidelines for water quality?
- What is the water quality at the inlet point?
Water treatment cannot be directly transferred from one location to another!
A comprehensive analysis from water treatment to the sterilization process is required to assess the overall process for water.
The Working Group for Instrument Reprocessing (AKI) has been engaged in a holistic examination of the instrument cycle for many years.

The accompanying brochure already contains notes and recommendations that are significantly below the threshold values of DIN EN 285.
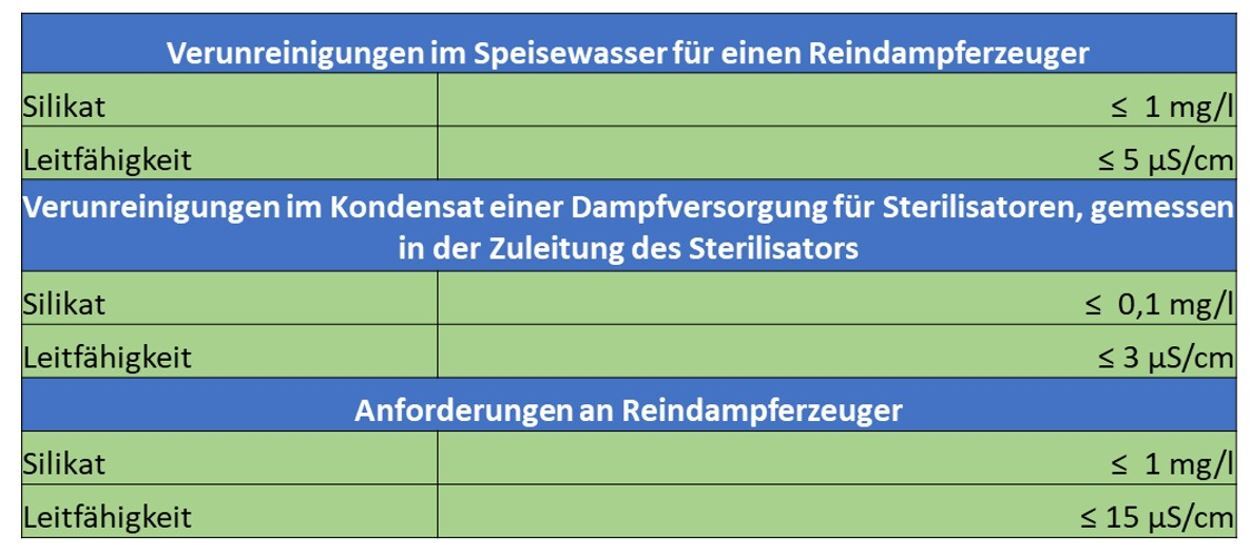
“The so-called silica slip already occurs at conductivities above 1 µS/cm!
To reliably achieve a value of less than 0.1 mg/l in the condensate, the feed water to the sterilizer should not contain more than 0.4 mg/l SiO2!”
To consistently obtain spot-free instruments, the silica content should permanently be below 0.4 mg/l.
Quality monitoring of the fully demineralized water through electrical conductivity control is not sufficient for identification, as the silica does not impart conductivity to the water.”
Excerpt from the 11th edition 2017; Pages 21-22
The threshold values for drinking water differ significantly from the process-engineering permissible threshold values. At the same time, the chemical composition or the proportion of dissolved substances in water varies depending on the water source. Therefore, to comply with the required threshold values, water treatment must be specifically tailored to the raw material “drinking water”.
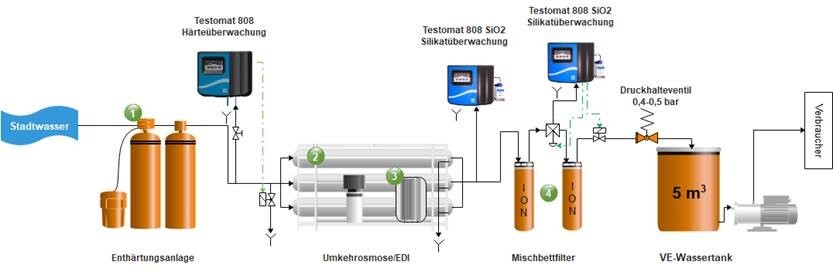
The conversion of drinking water into feed water for a steam generator typically involves the following process stages:
- Water softening system
- Reverse osmosis
- Electrodeionization
- Mixed-bed
- Number of Cleaning and Disinfection Devices (RDG)
- Number of Steam Sterilizers
- Number of Container Washing Units (CWA)
- Operating model of the facility (e.g., shift operation of 12, 16, or 24 hours)
All mentioned units have specific power consumption per hour and specific running times in minutes (depending on the sterilization program and device type)!
If additional consumers are supplied by the same water treatment system, the water demand of these consumers must also be recorded.
From the recorded data, initial relevant process parameters can be approximately determined:
- Maximum consumption rate per minute (among other things relevant for the design of pressure boosting)
- Daytime demand for deionized water (relevant for the design or evaluation of existing storage tanks)
Based on these quantities, an initial estimation of the existing system concept is already possible.
Now, based on these quantities, a backward calculation is performed for the individual stages of water treatment. The following questions must be taken into account:
- For what period of time must emergency supply from the permeate tank be ensured at least?
- What is the maximum consumption rate per minute? In this consideration, the approach with a simultaneity factor of 90% has proven effective (90% of all existing consumers demand water at the same time).
- Which switching points of the level switch must be considered for replenishment? The goal is to find a balance between consumption and replenishment, ensuring that the systems run for a sufficient duration but do not have excessively long downtime.
- What yields do the individual modules provide? In each treatment stage, there is a wastewater stream. The “loss quantities” must be considered in the system performance.
- What is the composition of the raw water before the first treatment stage? The water analysis provides important information for the selection of suitable treatment stages and the selection of associated components. For example, water hardness directly influences the dimensioning of the water softening system. The output performance of the water softening system, in turn, is crucial for the selection of reverse osmosis and thus also electrodeionization (EDI). Other relevant constituents may include disinfectants and dissolved heavy metals.
Is the existing water treatment technology suitable for meeting the current demand for process water and potentially ensuring the already known future demand?
In conclusion, all individual components involved in the process:
- Process water quality
- Pipeline systems
- RDG/CWA devices
- Auxiliary equipment such as steamers and ultrasonic devices
- Cleaning chemicals
- Steam sterilizers
- Pure steam generators
- Feeding technology
are crucially influenced by the quality of the feed water and steam quality. If this is not correct, a stable and safe processing process cannot take place. In addition to the quality of the process water, the device technology and the structure of the medical device processing unit (AEMP) also play a crucial role in the sterile goods processing process.
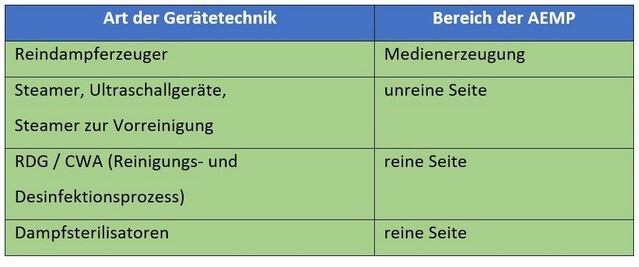
What equipment technology is required in the AEMP for sterile processing?
During reprocessing, the following essential tasks are carried out by trained personnel:
- Recording of delivered medical devices in the ERP system
- Pre-cleaning of the instrumentation to remove adherent impurities and deposits; if necessary, disassembly for the reprocessing process
- Loading of transport devices for the mechanical cleaning and disinfection process in the RDG and/or CWA
- Functional testing and assembly including packaging in sterile barrier systems (containers, wraps, etc.) and, if necessary, restocking from the sterile goods store
- Sterilization
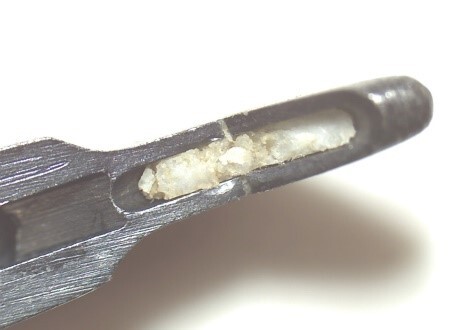
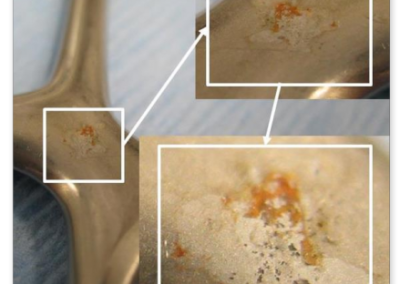
Example of insufficient cleaning performance of the RDG (cartilage residues)
In the well-known specialist literature, there are no clear, comprehensive regulations. The DGSV publishes extensive recommendations, which are created and updated with the participation of experts in the respective fields. Among other things, the following publications have already been made for the construction or renovation of an AEMP:
Requirements for hygiene in the reprocessing of medical devices
- Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM), 10/2012, in particular Annex 5 “Overview of Requirements for Reprocessing Units for Medical Devices”
- Recommendation for the reprocessing of medical devices, framework conditions for uniform administrative action. Created by: Project group “RKI-BfArM recommendation” of the Medical Devices Working Group (AGMP)
- DIN 1946:4, 2008 “Ventilation and air conditioning systems in healthcare buildings” – TRBA 250 – 2014
as well as
- Years of experience
The focus of the recommendations lies in the structural prerequisites.
In practice, deficiencies in sterilization often stem from insufficient spatial separation within an AEMP and inadequate involvement of users in the planning processes. Deviations from the above recommendations may arise from the specific conditions within healthcare facilities themselves or from the particular requirements within the AEMP.
Hygiene regulations and, if applicable, the monitoring regulations of the states must be observed, e.g., regarding the involvement of the hygiene commission in structural or organizational changes with hygiene relevance.
In general, three areas and additional rooms are required:
- CLEANING AND DISINFECTION AREA (unclean)
- Acceptance zone, possibly with PC workstation
- Manual pre-cleaning work area
- Cleaning/disinfection area
- Possible separation of cleaning/disinfection routes for thermostable/thermolabile items
- RDG loading zone
- Possible trolley washing area/trolley washing system
- Staging area for transport/loading trolleys
- Possible staging area for RDG loading trolleys
- If vertically connected
- Access to the elevator “unclean”
- Adjacent rooms, possibly dosing room (dosing center), possibly utility room
- PACKING AREA (clean)
- RDG removal zone with approval
- PC workstation
- Intermediate storage
- Packing and inspection stations, possibly with PC workstation
- Possible separate workstations for low-contamination/sterilizable goods, different packaging materials/sterilization methods
- Possible staging area for loading trolleys and/or transport racks
- Sterilizer loading zone
- Adjacent rooms, such as utility room and possibly storage room for replacement instruments, consumables/supplementary materials
- STERILE GOODS AREA (clean)
- Sterilizer removal zone
- Cooling area with approval, possibly with PC workstation
- Picking
- Possible storage zone and dispensing area
- If vertically connected
- Access to the elevator “clean”
Apart from the structural requirements, ensuring proper sterilization requires the flawless functioning of the equipment used. With water being used in the form of steam and ultrapure water for cleaning processes, the surfaces are constantly in contact with it. Water treatment tailored to the process can ensure the required quality of the media. Considerations must also be made for the media distribution system, especially regarding the piping systems.
A common cause of recontamination of properly treated water is contamination in the pipelines, which can arise from factors such as:
- improper installation (e.g., hot cutting instead of cold cutting, lack of deburring, unsuitable materials, etc.),
- inadequate maintenance of equipment, and
- improper dosing of cleaning agents.
This leads to additional costs for equipment and media line repair/maintenance. Specifically, the costs for refurbishing the pipelines and for treating the equipment are as follows:
- Chamber cleaning of a sterilizer: approx. 10,000€,
- Costs for equipment replacement,
- Container washing machine: 90,000€,
- Cleaning and disinfection device: approx. 60,000€,
- Pure steam generator: approx. 25,000€,
- Sterilizer (12 STE): 70,000€,
- Loading trolley: 2,500€ – 9,000€.
These expenses add up.
When these costs are applied to an exemplary clinic model, the following cost model for processing and replacing contaminated equipment and feed technology emerges.
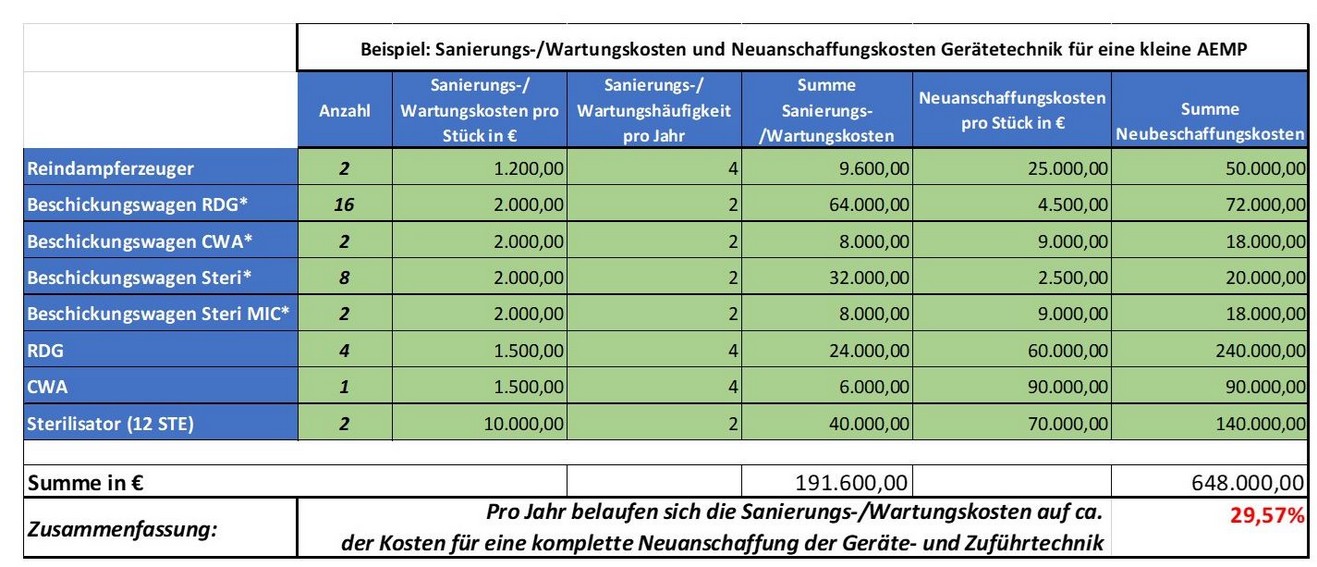
Minimizing avoidable risks is crucial to ensuring the highest availability of instrumentation and thus site and patient safety.
The processing of used instruments is significantly influenced by their handling in the operating room. Besides damages during use, there are other factors that can directly affect the subsequent treatment:
- The placement in the instrument tray
- The exposure or contact with substances from the OR area (e.g., saline solutions, ring solutions, etc.)
- The application of enzyme foam
- The duration until the instrumentation is sterilized
The specialized personnel in the processing unit inspect the incoming sterile goods on the unclean side and check for any deviations. If necessary, the instruments are disassembled here and thoroughly pre-cleaned to prepare them for the subsequent cleaning, disinfection, and sterilization processes.
In summary, a comprehensive approach in the sterilization process includes implementing preventive measures through comprehensive risk management and identifying potential savings.
Particular attention is paid to the preservation of medical devices. Targeted measures are taken to minimize acquisition costs, repair costs, and replacement costs. These include optimizing the tray system to reduce procurement volume and weighing the decision between repair and replacement. Important indicators include the service life of instrument groups in connection with the costs of processing, repair, and replacement.
Based on these aspects, the condition of instrument processing can be reliably assessed without the need for an inspection of the processing unit. A comparison of repair and replacement costs based on previous year’s data allows for an evaluation of whether costs are within the expected range or if there has been excessive cost escalation.
The creation of basic data for informed decision-making and the establishment of quality standards to ensure instrument and patient safety as well as compliance with legal requirements are at the forefront here.
The collection, interpretation, and evaluation of various data sets require comprehensive knowledge of the entire process, including the process engineering evaluation of systems, dosing, and other processing techniques. Close collaboration with procurement and controlling is also essential. In practice, this diversity of topics and responsibilities poses a challenge.
Our experts have extensive practical experience in analyzing and moderating processes in sterilization. Based on this, a tailored service offering has been developed to ensure comprehensive support for this complex matter. Particularly noteworthy are the building blocks outlined below, which can be used according to the needs and desires of the customer. To complement our service offering, we collaborate with accredited laboratories to conduct accompanying laboratory investigations, thus ensuring independence in consultation.
Our services
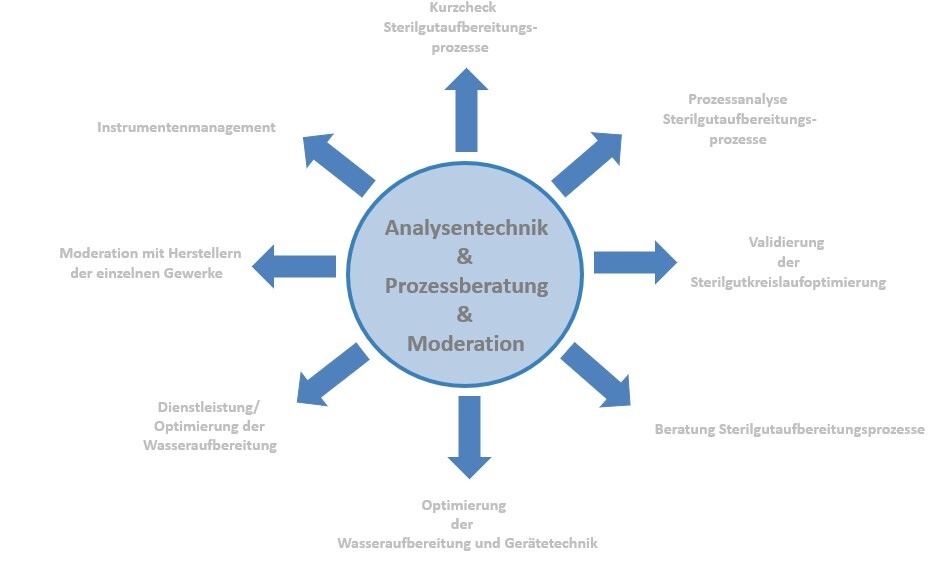
- Short check-up of sterilization processes
-
Process analysis of sterilization processes
-
Validation of the optimizations carried out in the sterilization cycle
-
Consultation and monitoring of optimization processes in the sterilization cycle
-
Training sessions and workshops on sterilization processes
We analyze, evaluate, and document the current status of water and steam preparation.
We inspect the sterilization processes and the quality of the instruments.
We develop tailored recommendations.
We support the implementation of these recommendations through our experts in water treatment, sterilization, and instrument management.
From this, measures for optimizing the processes emerge, which vary depending on the size of the facility and the desired level of process security. Our services aim to ensure the quality of instruments and processes permanently, thus ensuring patient safety.
It is also advisable to introduce targeted training measures to ensure the required quality and cost-effectiveness.
The optimization potential of a clinic depends mainly on the current quality situation in the areas mentioned.
The consistent implementation and monitoring of all measures can lead to a significant improvement in process security. Proper implementation and validation of measures are particularly important to ensure results.
This results in quality criteria for assessing existing hygiene measures. Recommendations are provided for their integration into existing safety measures, operating instructions, and procedures in the sterilization and hygiene process.
To ensure the necessary safety in the sterilization process, various measures in water treatment, instrument logistics, operating room, equipment technology, and general preparation must be implemented.
The safety and cost-effectiveness of the entire hygiene and sterilization process are achieved through process analysis and the integration of new technologies and working methods.
Summary
The costs associated with non-hygienic medical devices arise not only from direct acquisition, repair, and replacement costs but also from the time spent sterilizing the instruments according to applicable guidelines. Moreover, non-monetary costs such as compromising patient safety play a significant role in evaluating the sterilization process. Therefore, it is crucial to continuously maintain the performance of the entire sterilization system. Professional monitoring of water treatment and early detection of performance impairments can prevent damage and reduce downtime and revenue losses.
Analyzing and evaluating the existing sterilization process, developing a tailored concept for sustainable instrument preparation, and deriving recommendations for all involved parties are critical. This includes, for example:
- Optimizing process water treatment,
- Optimizing the media-carrying piping systems,
- Optimizing equipment and feed technology,
- Optimizing chemical usage,
- Optimizing sterilized goods logistics,
- Optimizing the sieve structure,
- Optimizing replenishment reserves,
- Training measures for personnel in the preparation unit and operating room area.
Based on the individual concept proposals, measuring and monitoring devices are installed at the water treatment plant, and measures in sterilized goods logistics and operating departments are defined, depending on the desired goals regarding efficiency, safety, and cost-effectiveness.
Minimizing avoidable risks is crucial to ensuring the highest availability of instruments and thus for site and patient safety.